Hydrogen fuel cell discovery by UC Berkeley dramatically reduces cost

A new technology to make hydrogen fuel cells last longer has been engineered by a University of California, Berkeley chemist.
Shannon Boettcher and his team are developing a new electrolysis technology using ion-conducting polymers, which would dramatically reduce costs and give hydrogen a new competitive power in the fuel industry.
“If you can make this really work, it’s not unreasonable to expect a 5x or 10x reduction in the cost of these membrane electrolysers, which would truly enable us to put them on the grid as a variable-load offtake of low-cost electrons and deliver hydrogen,” said Boettcher.
How does it work?
A new alternative to common electrolyser types is a proton exchange membrane (PEM) electrolyser. This uses an acidic ion-conducting organic polymer membrane to serve as the electrolyte, and keeps the oxygen and hydrogen gases apart.
“This electrolyser is beautiful because the membrane blocks to a much larger extent the oxygen and hydrogen from mixing across this membrane,” said Boettcher.
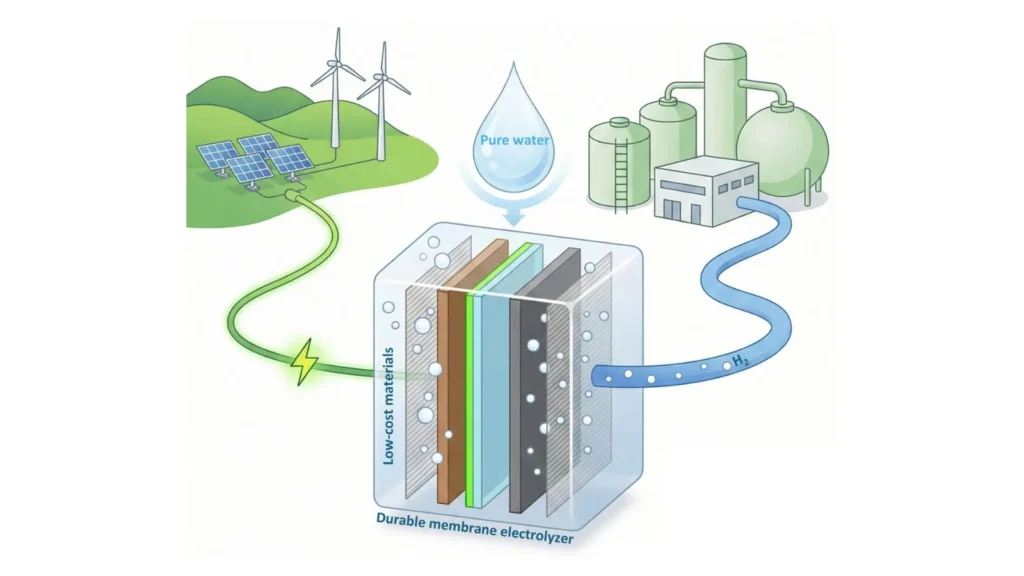
“You can get the two electrodes very close together and make hydrogen at one electrode at high efficiency, oxygen at the other electrode at high efficiency, and the membrane is the electrolyte – the salt solution – but it’s a solid so you only need to feed it pure water.”
However, these electrolysers use the extremely expensive metal iridium – any other metal will dissolve in the strongly acidic environment in the cell.
Boettcher’s new technology, called an anion-exchange-membrane water electrolyser, combines the advantages of a solid polymer membrane with the low cost of commercial types of electrolysers.
Boettcher and his team devised a way to protect the polymer from degradation by using a zirconium oxide inorganic polymer – an inexpensive ingredient, and certainly cheaper than iridium.
“You can get all the low-cost material advantages of one technology – the alkaline technology – with all the advantages of the membrane technology, including lower cost, higher safety and less maintenance, but to do that they need to be durable,” he said.
Who is behind the discovery?
Boettcher is the founding director of the Center for Electrochemical Science, Engineering and Technology (CESET) at UC Berkeley, and runs a lab researching electrochemistry and material science.
Coauthors on this research, published in the journal Science, include Shujin Hou, Yang Zhao, Minkyoung Kwak, Kelvin Kam-Yun Li, Peiyao Wu, Anthony Ekennia and Joelle Frechette of UC Berkeley and Gregory Su of Berkeley Lab. There are collaborators at Versogen, a Delaware based company working to commercialise the technology, the University of Delaware and Stanford.
This current work is funded by the US Department of Energy.
What happens next?
Boettcher hopes that improved electrolysers and other new battery technology will receive a boost from a $28 million investment California made in the commercialisation of electrochemical technologies earlier this year.
The California Energy Commission awarded the money to the Electrochemistry Foundry in Hayward, which UC Berkeley is partnering with to provide internship opportunities.
“Hydrogen production, storage, shipping – they’re all expensive and there’s a lot of challenges. But the progress in this technology is incredible,” Boettcher said. “The era of hydrogen fuel from electrolysis outcompeting fossil fuels without subsidy for many different applications is coming.”